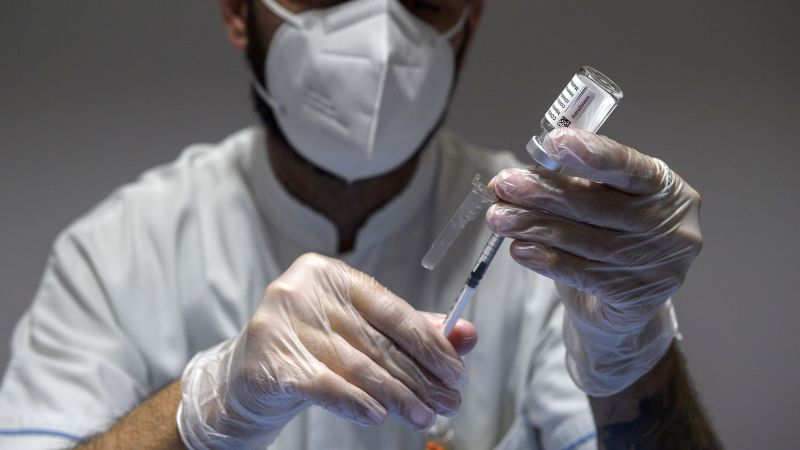
In June 2021, a healthcare worker was seen preparing doses of the AstraZeneca Covid-19 vaccine at a vaccine hub in Rome. Developed in partnership with the University of Oxford, Vaxzevria has been widely administered worldwide, with over 3 billion doses supplied since January 4, 2021. However, despite its global reach, the company has announced the withdrawal of Vaxzevria due to declining demand.
AstraZeneca attributed the decline in demand for Vaxzevria to the surplus of new Covid-19 vaccines available on the market. As a result, the company ceased manufacturing and supplying the product. This decision led to AstraZeneca initiating the withdrawal of marketing authorizations for Vaxzevria within Europe. The European Medicines Agency also announced the withdrawal of Vaxzevria’s marketing authorization in EU countries.
Despite this discontinuation, AstraZeneca remains proud of its role in ending the pandemic through its successful coronavirus vaccine. This is a developing story and updates will be provided as more information becomes available.